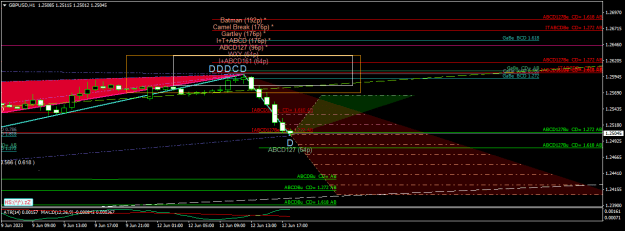
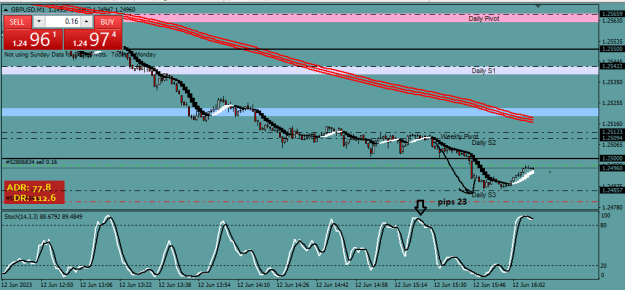
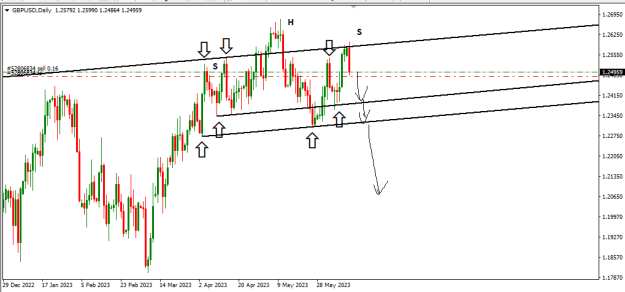
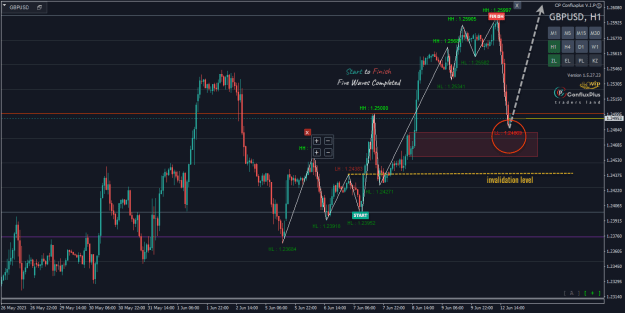
Disliked{quote} Now read that article carefully and copy-paste here the part where it says they melted something into graphene.Ignored
By pyrolyzing the material on the substrate, carbon clusters are prevented from forming. Due to the amount of energy required to break the carbon bonds (C-C = 347 kjmol-1, C=C = 614 kjmol-1, C≡C = 839 kjmol-1, C-H = 413 kjmol-1), a high heat is required, and therefore a metal catalyst is required during the process.
The second step is a heat intensive step which assembles the dissociated carbon atoms onto a substrate (in the presence of a catalyst), which forms a single layer structure.
Pyrolyzing - thermal decomposition and thermal assembly as opposed to simple melting
1
2